Natural Product Synthesis
Natural Product Synthesis – Chemical Discovery through the Pursuit of Complex Molecular Scaffolds
The general research paradigm within the Stoltz laboratory is to utilize architecturally complex target molecules as the driving force behind the development of new reactions. Naturally, these endeavors continuously push the boundaries of known chemical reactivity, highlighting the limitations of current technologies. The ensuing synthetic effort represents not only a feat of synthetic strategy, but one of creativity and ingenuity.
Check out these recently completed projects:
(+)-Daphnepapytone A – Chem. Sci. 2025
(–)-Crotonine G and (–)-Crotonolide D – J. Am. Chem. Soc. 2025
Lycojapomine A and B – J. Am. Chem. Soc. 2025
(–)-Cylindrocyclophane A – Science 2024
Hypersampsone M – J. Am. Chem. Soc. 2024
Makaluvamines A, C, D, and N, and isobatzelline B – Chem. Sci. 2024
(–)-Hunterine A – J. Am. Chem. Soc. 2024
Aleutianamine - J. Am. Chem. Soc. 2023
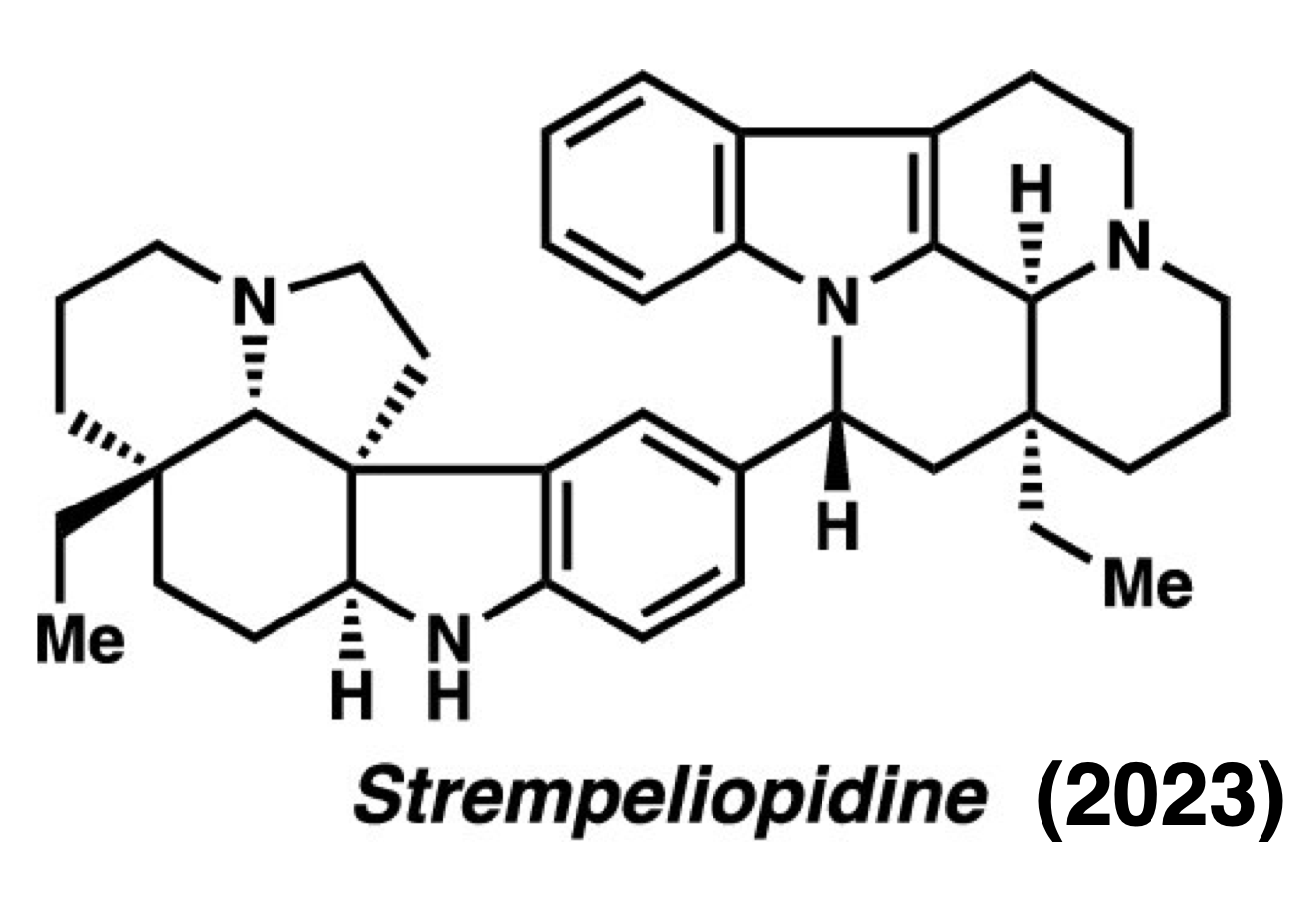
Strempeliopidine – J. Am. Chem. Soc. 2023
(–)-Yonarolide – Chem. Sci. 2023
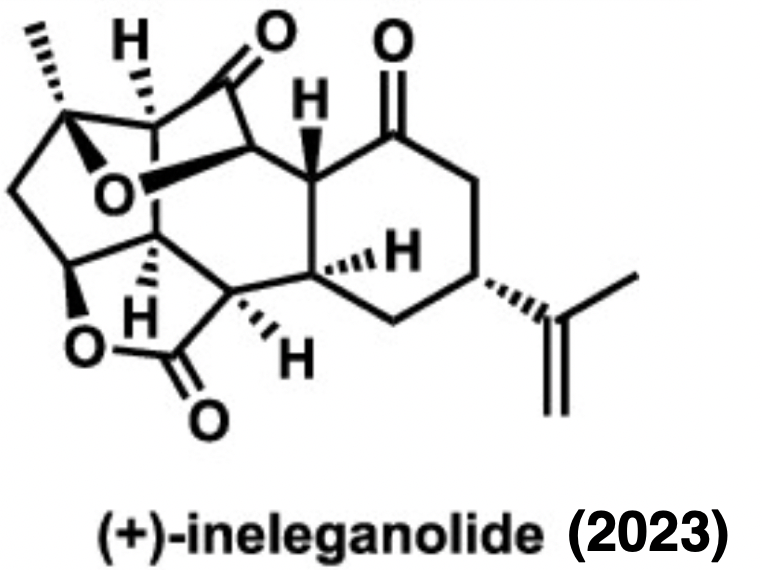
(+)–Ineleganolide – J. Am. Chem. Soc. 2023
Havellockate – J. Am. Chem. Soc. 2022
Eburnamonine, Eucophylline, and 16′-epi-Leucophyllidine – Angew. Chem. Int. Ed. 2021
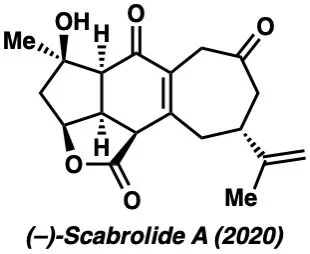
(–)-Scabrolide A – J. Am. Chem. Soc. 2020
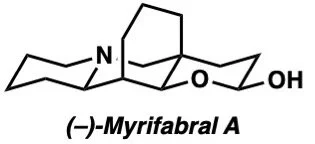
(–)-Myrifabral A and B – Chem. Sci. 2020
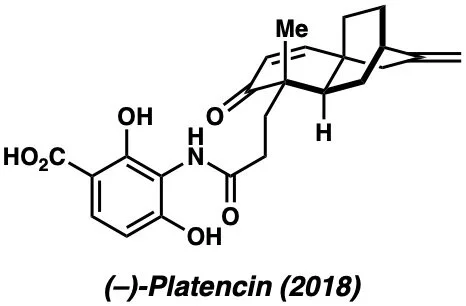
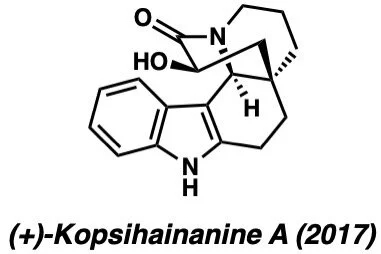
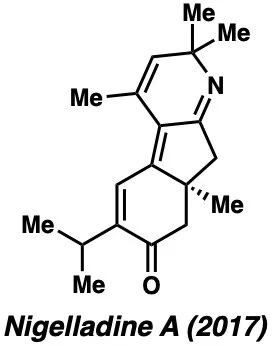
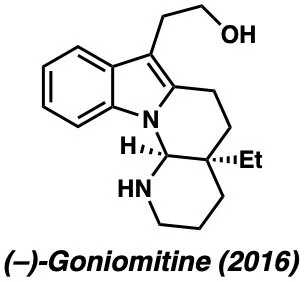
Below are examples of molecules previously synthesized by our group: